
The classical model of hematopoiesis, which is based mainly on populations defined by flow cytometry 15, 16, 17, has recently been challenged in several aspects by single-cell transcriptomic 9, 10, 18, 19, 20, functional 21, 22 and lineage tracing 23 approaches.

The differentiation of hematopoietic stem cells (HSCs) in the bone marrow (BM) constitutes a particularly striking example of this disconnect 11, 12, 13, 14. Together, these findings highlight a disconnect between single-cell genomics-based molecular cell type maps and data generated by widely used cytometry assays. Conversely, the precision and efficiency of commonly used cytometry gating schemes are largely unknown, and the exact importance of many surface markers remains unclear. Hence, single-cell transcriptomics (scRNA-seq) approaches have demonstrated that flow cytometry gating schemes frequently yield impure or heterogeneous populations 9, 10, and flow strategies for the precise identification of cell types defined by scRNA-seq are lacking.
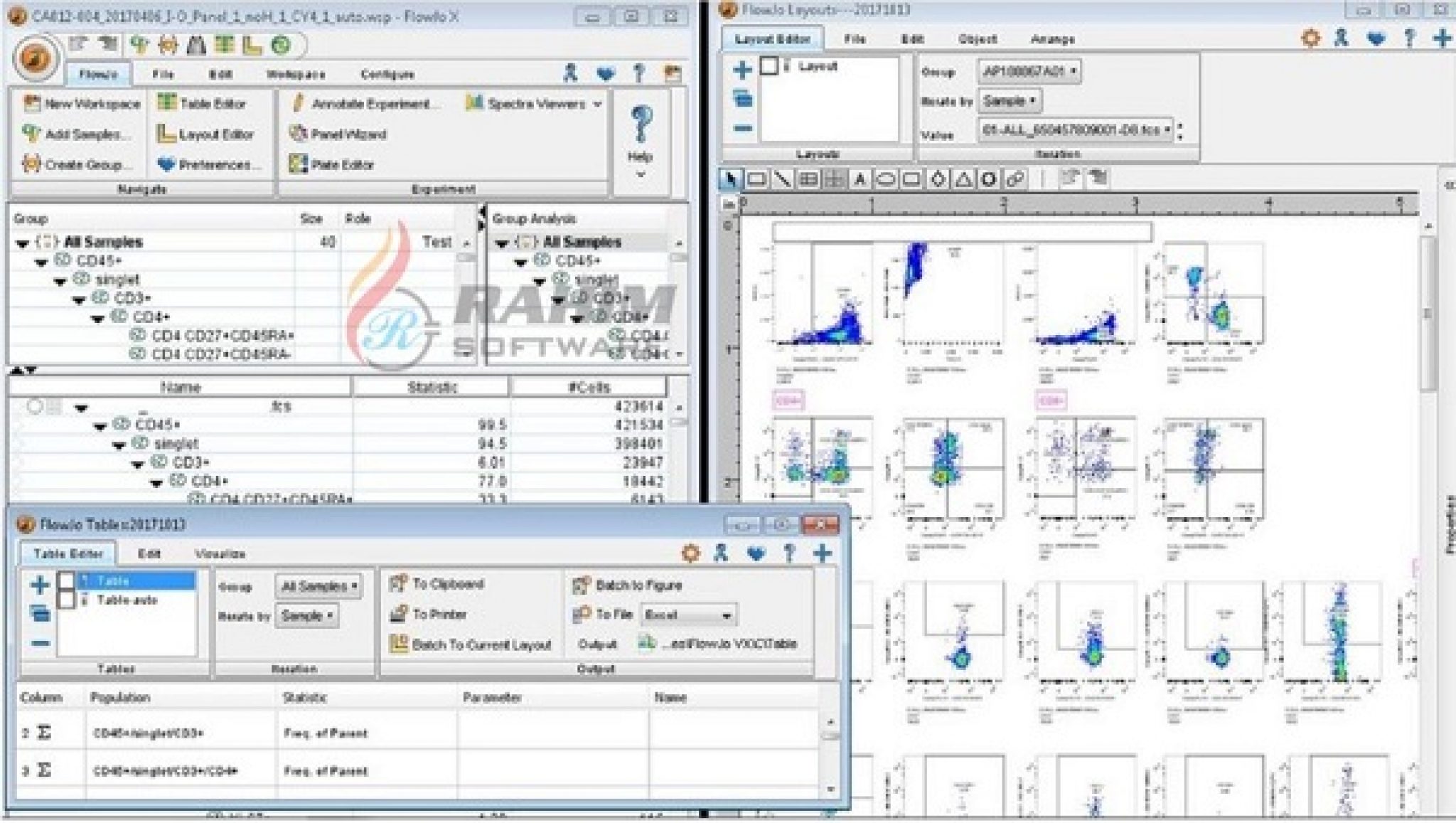
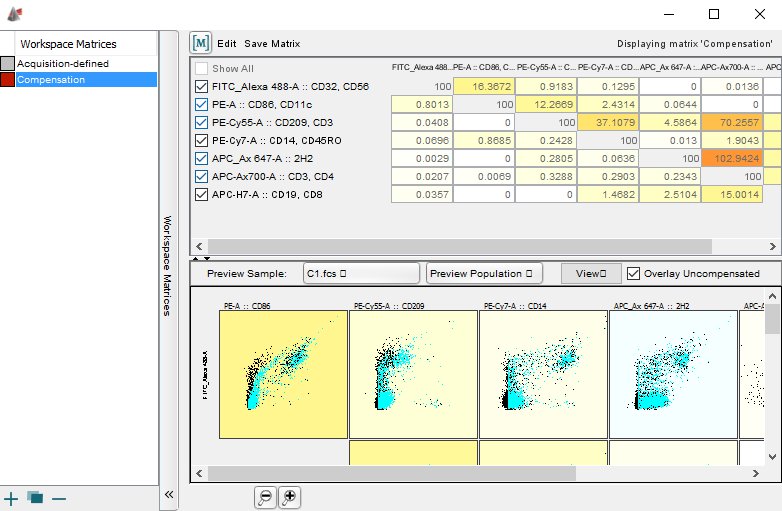
#FLOWJO REFERENCE TRIAL#
However, flow cytometry provides low-dimensional measurements and relies on predefined sets of surface markers and gating strategies that have evolved historically in a process of trial and error. Unlike single-cell transcriptomics, flow cytometry offers a massive throughput in terms of samples and cells, is commonly used in routine clinical diagnostics 8 and remains unrivaled in the ability to prospectively isolate live populations of interest for downstream applications. Furthermore, single-cell genomics technologies remain cost-intense and scale poorly, impeding their integration into clinical routine. However, strategies for the prospective isolation of cell populations newly identified by single-cell genomics are needed to enable their functional characterization or therapeutic use.
The systematic construction of whole-organ and whole-organism single-cell atlases has revealed an unanticipated diversity of cell types and cell states, and has provided detailed insights into cellular development and differentiation processes 4, 5, 6, 7. Single-cell transcriptomic technologies have revolutionized our understanding of tissues 1, 2, 3. Our study serves as an accessible resource and paves the way for a data-driven era in cytometry. The systematic integration of cytometry and proteo-genomic data enables the functional capacities of precisely mapped cell states to be measured at the single-cell level. These reference maps enable the automatic design of cost-effective high-throughput cytometry schemes that outperform state-of-the-art approaches, accurately reflect complex topologies of cellular systems and permit the purification of precisely defined cell states. Here, we have generated high-content single-cell proteo-genomic reference maps of human blood and bone marrow that quantitatively link the expression of up to 197 surface markers to cellular identities and biological processes across all main hematopoietic cell types in healthy aging and leukemia.
However, excessive cost and a lack of strategies for the purification of newly identified cell types impede their functional characterization and large-scale profiling. Single-cell genomics technology has transformed our understanding of complex cellular systems. Nature Immunology volume 22, pages 1577–1589 ( 2021) Cite this article Single-cell proteo-genomic reference maps of the hematopoietic system enable the purification and massive profiling of precisely defined cell states


 0 kommentar(er)
0 kommentar(er)
